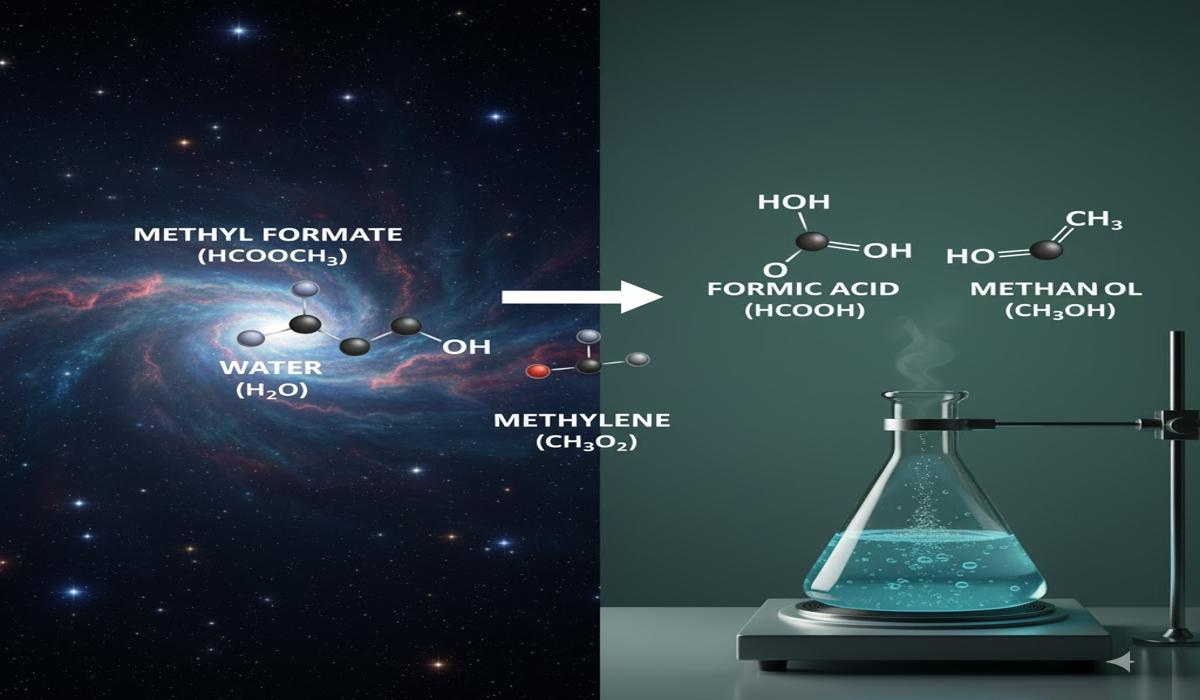
In the fascinating world of chemistry, even the smallest molecules can play mighty roles. When we examine compounds like HCOOCH (methyl formate), CH₂ (methylene), and H₂O (water), we step into a captivating area of organic and aqueous chemistry that has wide-reaching implications for biology, environmental science, astrochemistry, and industrial synthesis.
These three simple molecules might appear unrelated at first glance, but together they represent a remarkable trio that bridges earthbound chemistry and cosmic processes. From the synthesis of organic esters in laboratories to the detection of molecules in interstellar space, HCOOCH, CH₂, and H₂O embody the interconnectedness of chemical principles across scales, both microscopic and astronomical.
Breaking Down the Trio: HCOOCH, CH₂, and H₂O
1. HCOOCH – Methyl Formate
Methyl formate (commonly written as HCOOCH₃) is the methyl ester of formic acid. It has the following characteristics:
- Chemical Formula: C₂H₄O₂
- Structure: Formed by the esterification of formic acid (HCOOH) with methanol (CH₃OH).
- Physical Properties: A colorless, volatile liquid with a fruity odor, often compared to rum or raspberries.
- Occurrence: Found both in terrestrial environments (factories, fruits, and solvents) and in interstellar space, where it has been detected in star-forming regions.
Methyl formate is important because it can undergo hydrolysis in aqueous solutions: HCOOCH3+H2O→HCOOH+CH3OHHCOOCH₃ + H₂O \rightarrow HCOOH + CH₃OHHCOOCH3+H2O→HCOOH+CH3OH
This reaction produces formic acid and methanol, both of which are valuable chemicals in biology and industry.
2. CH₂ – Methylene
Methylene (CH₂) is a highly reactive carbene. It is rarely found in its free form due to extreme instability but plays a central role in organic reactions.
- Chemical Identity: Carbene with the formula CH₂.
- Reactivity: Extremely reactive because it has two nonbonded electrons on carbon.
- Forms: Exists in singlet and triplet states, with triplet methylene being more stable.
- Importance in Chemistry: Involved in cycloaddition reactions, insertion reactions, and polymerization.
Although methylene is not as stable or commonly isolated as methyl formate or water, its fleeting presence enables the formation of complex molecules from simple starting materials. It’s one of the hidden engines behind organic synthesis.
3. H₂O – Water
Perhaps the most familiar of the trio, water (H₂O), is the universal solvent and the medium of life.
- Chemical Formula: H₂O
- Polarity: Strongly polar, allowing it to dissolve countless ionic and polar compounds.
- Biological Role: Foundation of all known life processes, from metabolic reactions to structural organization of cells.
- Cosmic Presence: Found throughout the universe, from comets to planetary atmospheres.
Water plays a key role in the hydrolysis of methyl formate, facilitating the breakdown of esters into their acid and alcohol counterparts. It’s the bridge that connects organic molecules to functional transformations.
The Interplay Between HCOOCH, CH₂, and H₂O
At the crossroads of organic and aqueous chemistry, these three compounds demonstrate how small molecules interact to drive larger processes. Let’s examine a few contexts where their interplay becomes important:
- Ester Hydrolysis (HCOOCH + H₂O)
- Acid or base catalyzed, the hydrolysis of methyl formate in water yields formic acid and methanol.
- This simple yet profound reaction is fundamental in both biological metabolism and chemical engineering.
- Methylene and Ester Reactions (CH₂ with HCOOCH)
- Though methylene is fleeting, it can react with esters like methyl formate in complex pathways.
- These reactions are relevant in synthetic organic chemistry for building larger carbon frameworks.
- Astrochemical Relevance
- Methyl formate has been observed in interstellar dust clouds, where water and reactive intermediates like methylene also exist.
- This suggests that the trio might contribute to prebiotic chemistry, the chemical groundwork for life.
Industrial and Scientific Importance
Industrial Applications of Methyl Formate
- Used as a solvent in coatings and adhesives.
- Precursor to formamide, which is essential in producing pharmaceuticals, pesticides, and resins.
- Environmentally, it is considered a greener blowing agent in foam production, replacing ozone-depleting substances.
Role of Methylene in Organic Chemistry
- Key player in the Wolff rearrangement, cyclopropanation, and various insertion reactions.
- Helps chemists build complex molecules from simple starting materials.
- Though it cannot be bottled and stored, its in-situ generation makes it invaluable in synthesis.
Importance of Water in Chemistry and Beyond
- Essential solvent for acid-base reactions, redox processes, and hydrolysis.
- Medium for life’s biochemistry, from DNA replication to protein folding.
- Industrially critical for cooling, cleaning, dissolving, and transporting chemicals.
The Cosmic Connection: Chemistry Beyond Earth
One of the most fascinating aspects of HCOOCH, CH₂, and H₂O is their astrochemical significance. Astronomers using radio telescopes have identified methyl formate in interstellar clouds such as Sagittarius B2, one of the largest molecular clouds near the center of the Milky Way.
In such environments:
- Water ice coats dust particles.
- Energetic radiation creates reactive intermediates like methylene.
- Reactions occur on dust grain surfaces, forming esters, alcohols, and acids.
This demonstrates how the same chemical principles observed in labs are also operating in the vastness of space, contributing to the potential origins of life.
Environmental and Biological Insights
- Formic Acid (from hydrolysis of methyl formate): Found in ant venom and plant defenses, influencing ecosystems.
- Methanol: A simple alcohol used by some bacteria as an energy source.
- Reactive Carbenes: Though short-lived, intermediates like methylene reveal how life could assemble complex molecules under prebiotic conditions.
- Water Cycle: Ensures transport, reactions, and stability for countless chemical processes on Earth.
Together, these molecules highlight the continuity of chemistry: from Earth’s ecosystems to the industrial lab bench, and out into interstellar space.
Frequently Asked Questions (FAQs)
Q1: What is the chemical formula of methyl formate?
A: The chemical formula is HCOOCH₃ (C₂H₄O₂). It is the methyl ester of formic acid.
Q2: How does methyl formate react with water?
A: It undergoes hydrolysis: HCOOCH3+H2O→HCOOH+CH3OHHCOOCH₃ + H₂O \rightarrow HCOOH + CH₃OHHCOOCH3+H2O→HCOOH+CH3OH
producing formic acid and methanol.
Q3: Why is methylene (CH₂) important if it is unstable?
A: Methylene acts as a reactive intermediate in many organic reactions, making it essential in the formation of complex molecules.
Q4: Where is methyl formate found in space?
A: It has been detected in star-forming regions like Sagittarius B2, suggesting that esters can form in interstellar environments.
Q5: What role does water play in these reactions?
A: Water serves as a solvent, reactant (in hydrolysis), and stabilizing medium, making it central to both Earth-bound and cosmic chemistry.
Q6: Are these molecules relevant to biology?
A: Yes. Formic acid (from methyl formate) is found in insects, methanol is metabolized by microbes, and water is the basis of all known life.
Conclusion
The trio of HCOOCH (methyl formate), CH₂ (methylene), and H₂O (water) represents an exciting crossroad of organic, aqueous, and astrochemistry. From laboratories to the vast reaches of space, these molecules remind us that even the smallest building blocks can yield profound insights into life, industry, and the universe itself.
Their interactions—whether in the hydrolysis of an ester, the fleeting dance of a carbene, or the solvent power of water—illustrate the unifying beauty of chemistry. By studying them, we deepen our understanding of not only matter itself but also the processes that connect Earth to the cosmos.